What Is Ultraviolet Spectroscopy? The Interesting Answer!
Last Updated on

Spectroscopy refers to the interaction of light with matter. In ultraviolet spectroscopy, UV light is used to study the properties of molecules. It can help determine molecules’ structure, composition, and interaction with other molecules.
Ultraviolet spectroscopy is a powerful tool that is used in a variety of fields. Some of them include medicine, environmental science, and forensics. For example, it can be used to identify chemicals, study how they interact with each other, and determine their structure.
Since UV light ranges from 100 nm (nanometers) to 400 nm, ultraviolet spectroscopy uses a range of light invisible to the human eye.1 It means that special equipment is needed to observe and study the effects of UV light on molecules.
Let’s take a closer look at UV spectroscopy, its benefits, and its uses in the industry.

How Does It Work?
Before specifically understanding how UV spectroscopy works, you need to understand the basic principle of spectroscopy.
In spectroscopy, light is used to study the properties of molecules. The light interacts with the electrons in the molecule. The interaction can provide information about the molecule’s structure and composition. It can also reveal how the molecule interacts with other molecules.
In ultraviolet spectroscopy, UV light is used instead of visible light. Often, UV and visible light are combined in what is called “UV-visible spectroscopy.”
- The matter absorbs light. As a result, the atom’s energy increases.
- Due to this, the electrons in the atom are excited and move to a higher energy state.
- Molecules with nonbonding or π-electrons absorb UV light as energy. The absorption of light results in the electrons moving to the higher orbitals.
- The π-electrons are located in the p-orbitals of a molecule. These orbitals extend above and below the plane of the molecule. The π-electrons are more exposed to UV light than the other electrons in the molecule.
- The energy required to move an electron from the ground to the excited state is known as the “excitation energy.” The excitation energy is different for every molecule.
- The wavelength of the light absorbed is also different for every molecule. The wavelength of light a molecule absorbs depends on the molecule’s structure.
- The combination of the wavelength of light absorbed and the excitation energy can help determine the structure of a molecule.
Components of UV Spectroscopy
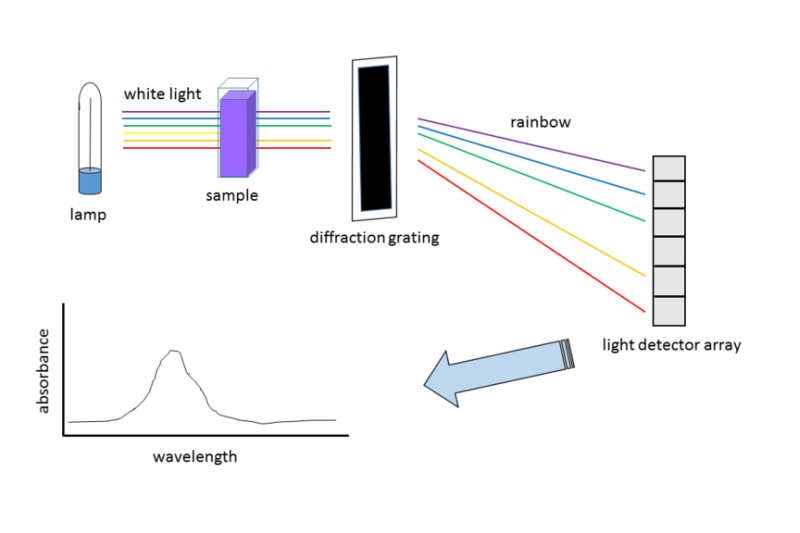
UV spectroscopy employs multiple instruments. Here are the most important ones:
Light Source
The light source is the first component of a UV spectroscopy setup. For instance, a standard light source is a tungsten lamp. Tungsten lamps emit light over the whole UV region.
Some applications may also use hydrogen-deuterium lamps. For example, while tungsten lamps mainly emit red radiations of 375 nm wavelength, hydrogen-deuterium lamps emit wavelengths below this range.
Monochromator
A monochromator is a device that selects a narrow range of wavelengths from a broadband light source. The monochromator helps in isolating the required wavelength for a particular analysis.
It is placed between the light source and the sample compartment. The light from the light source passes through the monochromator. The monochromator then filters the light and sends a beam of the required wavelength to the sample compartment.
Spectrophotometers are the monochromators in UV spectroscopy. Most of them are double-beam spectrophotometers. It means they have two light beams- one that passes through the sample and one that doesn’t.
The light that passes through the sample is called the “sample beam.” Meanwhile, the other is the “reference beam.”
The reference beam is a reference point to compare the sample beam. It helps eliminate any errors caused by the light source or the monochromator.
Sample and Reference Cells

Quartz or silica cells are commonly used in UV spectroscopy. The cells hold the solution. Since glass absorbs UV light, it is not applicable in this case.
Detector
The standard detectors in UV spectroscopy are photocells. While one photocell gets the beam from the reference, the other receives it from the sample.
The radiation intensity from the reference and sample are different since the reference has a stronger intensity. As a result, alternating currents generate in the photocells.
Amplifier
The alternating current from the photocell goes to the amplifier. The amplifier’s role is to make the current stronger. Thus, it helps make the current recordable.
Recorder
The recording device is the final component of a UV spectroscopy setup. The recorder helps in recording the current from the amplifier. The recorder can be either an X-Y plotter or a strip chart recorder.
An X-Y plotter records the current as a function of wavelength. Meanwhile, a strip chart recorder records the current as a function of time.

What Are the Different Types of UV Spectroscopy?

UV spectroscopy doesn’t have any types. However, it can be coupled with visible spectroscopy to create combined spectroscopy used to study organic compounds.
UV-Vis Spectroscopy
UV-Vis spectroscopy is a technique that combines the principles of both absorption spectroscopy and reflectance spectroscopy. By using a light source with a known wavelength, it is possible to determine how much of the light is absorbed or reflected by a sample.
The resulting data can help identify the sample’s chemical composition and information about its physical and chemical properties.
In this type of spectroscopy, a beam of light is directed at a sample. The sample absorbs some of the light and reflects the rest. The amount of light reflected or absorbed can be measured, and this data can be used to identify the sample’s chemical composition. Some typical applications of UV-Vis spectroscopy include:
- RNA and DNA Analysis: Researchers can use UV-Vis spectroscopy to study the absorbance of light by RNA and DNA. They use this information to understand the structure and function of these biomolecules.
- Pigment Analysis: UV-Vis spectroscopy can help identify the pigments in a sample. For example, if you were testing the color of a paint sample, you could use this technique to determine the type and amount of pigments present.
- Environmental Testing: UV-Vis spectroscopy also helps monitor environmental contamination. Experts use this technique to measure the absorbance of light by pollutants in water or air samples.
- Polymer Analysis: Polymers are large molecules made up of smaller units called monomers. UV-Vis spectroscopy can help study the absorption of light by polymers.
Where Is It Used?
UV spectroscopy has many applications. For example, you can use it in environmental testing to measure the number of pollutants in the air, water, and soil. It’s also used in medical diagnosis and treatment.
Here are some uses of UV spectroscopy.

Detecting Impurities in Pharmaceutical Settings
Organic molecules, such as drugs and pesticides, often have impurities. For example, a pharmaceutical company may want to ensure that a new drug is free of contaminants.
To do this, they take a drug sample and shine UV light. The light interacts with the molecules in the sample, and each type of molecule absorbs light at a different wavelength.
By looking at the absorbance spectrum, chemists can identify which molecules are present and in what quantities. Knowing this is important because impurities can change the way a drug works. They can also make it more toxic.
Structural Elucidation
It’s essential to know the structure of a molecule to understand its properties. How a molecule bends or twists can affect how it interacts with other molecules.
- Study Reaction Kinetics: The kinetics of a reaction are the rates at which the reactants are converted to products. To study reaction kinetics, researchers need to know the structure of the molecules involved in the reaction. UV spectroscopy helps them do that.
- Determine the 3D Structure of a Molecule: The 3D structure of a molecule affects its properties. For example, the 3D structure of a protein determines its function. Researchers use UV spectroscopy to determine the 3D structure of proteins, hormones, and other molecules.
- Detecting Functional Groups in Molecules: Functional groups impart properties to molecules. For example, the presence of a carbonyl group makes a molecule more reactive. Examples of functional groups are hydroxyl, carboxyl, and amine groups. UV spectroscopy helps detect these functional groups to study a compound’s properties.
- Molecular Weights: The molecular weight is the sum of the atomic weights of all the atoms in a molecule. It’s an essential property because it affects a molecule’s physical and chemical characteristics. Scientists prepare a compound derivative and use UV spectroscopy to measure its molecular weight.
Beverage Analysis
UV-Vis spectroscopy helps in the analysis of many types of beverages. For example, it can be used to determine the alcohol content of wine.
Likewise, there are legal limits on the amount of caffeine in soft drinks. UV-Vis spectroscopy can measure a drink’s caffeine content and ensure it falls within the legal limit.
Scientific Research
Scientific research employs UV spectroscopy in a variety of ways. For example, it’s used to study the structure of DNA and proteins. It is also helpful in cancer research, where researchers may have to determine the hemoglobin absorption in the body.
UV spectroscopy is also instrumental in air quality monitoring. For example, it can help measure the ozone, nitrogen dioxide, and sulfur dioxide levels in the atmosphere.
Advantages of UV Spectroscopy
UV spectroscopy has many advantages over other spectroscopic techniques. Here are some notable ones:
Highly Sensitive
UV spectroscopy is a very sensitive technique. Thus, it can detect tiny concentrations of molecules. Additionally, it can help study molecules that are difficult to detect using other methods.
Non-Destructive
UV spectroscopy is a non-destructive technique. It means that the sample is not damaged during the analysis.
Usually, other spectroscopic techniques, such as infrared spectroscopy (IR) or nuclear magnetic resonance spectroscopy (NMR), require the sample to be in a particular form (solid, liquid, or gas).
But UV spectroscopy can be used to study molecules in any state. As a result, there’s no need to prepare the sample in a particular way.
Wide Range of Applications
UV spectroscopy can help study many molecules, from small organic molecules to large proteins. Plus, it is helpful in various fields, such as medicine, chemistry, and biology.
Combination with Visible Spectroscopy
It is possible to combine UV spectroscopy with visible spectroscopy (Vis). The combination is called UV-Vis spectroscopy.
It can help scientists study a sample in more detail. Additionally, it can provide information about the sample that UV or Vis spectroscopy alone cannot give.
Disadvantages of UV Spectroscopy

Although UV spectroscopy is a powerful analytical technique, it also has some disadvantages.
May be Hazardous
UV light can be damaging to human tissue.
Therefore, the user has to be careful when handling UV light sources and using UV spectroscopy instruments.
May Require Specialized Equipment
Another disadvantage of UV spectroscopy is that it requires specialized equipment to generate and detect UV light.
The equipment can be expensive and may not be available in all laboratories. For instance, you need a UV light source, a spectroscopy cuvette, and a detector.
Time-Consuming
It takes a lot of time to prepare a sample for UV spectroscopy. For example, if you use a liquid sample, you must evaporate it until it becomes dry. The process can take several hours.
In addition, UV spectroscopy is a slow process. It can take several minutes to collect enough data for analysis.
Affected by Contaminants
Many external factors can affect the reading of the spectrometer. For example, the spectrometer might produce inaccurate readings if the area has any external light or electronic noise.
Contaminants in the air can also absorb UV light, which can distort the reading.

Frequently Asked Questions (FAQs)
Why Is Ultraviolet Spectroscopy Important?
UV spectroscopy has many applications in both research and industry. For example, in chemistry, UV spectroscopy can be used to identify unknown compounds, study the rates and mechanisms of chemical reactions, and investigate the structure of molecules.
In the pharmaceutical industry, it helps identify and quantify active ingredients in drugs. Meanwhile, in the food and beverage industry, UV spectroscopy helps monitor the quality of products and to detect contaminants.
What Is a Monochromator?
A monochromator is an instrument to select a single light wavelength (color) from a source with many different wavelengths. The most common type of monochromator used in ultraviolet spectroscopy employs a diffraction grating to separate light into its component wavelengths.
Which Instruments Are Used in UV Spectroscopy?
UV spectroscopy has the following components: light source, monochromator, detector, and data recorder. The light source emits light that passes through the monochromator. Meanwhile, the monochromator helps select specific wavelengths of light.
The detector then measures the amount of light that passes through the monochromator at each wavelength. An amplifier may also be present to increase the intensity of the current. It helps the recorder capture data more accurately. The recorder then plots the data on a graph.
How Does a Monochromator Work?
The light source is placed at the center of the grating. The light that passes through the grating separates into a spectrum. A slit allows only a narrow range of wavelengths to pass through to the detector, typically a photomultiplier tube (PMT) or a photodiode.
What Is a Spectrophotometer?
A spectrophotometer is an instrument to measure the amount of light a solution absorbs. The intensity of light that passes through a solution is measured. The results are plotted on an absorption spectrum or transmittance curve graph. The greater the absorbance, the less light passes through the solution.
What Is UV-Vis Spectroscopy?
In UV-visible spectroscopy, the interaction of light with matter in the ultraviolet and visible regions of the electromagnetic spectrum produces changes in light absorption, reflection, or transmission. The resulting spectrum can be used to identify unknown compounds and study the rates and mechanisms of chemicals.

Final Thoughts
Summing up, ultraviolet spectroscopy is a valuable technique that helps determine the properties of molecules. In particular, it can identify functional groups, determine the structure of a molecule, and investigate the interactions between molecules.
The components of a UV spectrophotometer are the light source, the sample holder, the monochromator, and the detector. Together, these components allow the applications of UV spectroscopy.
Featured Image Credit: Rabbitmindphoto, Shutterstock
About the Author Jeff Weishaupt
Jeff is a tech professional by day, writer, and amateur photographer by night. He's had the privilege of leading software teams for startups to the Fortune 100 over the past two decades. He currently works in the data privacy space. Jeff's amateur photography interests started in 2008 when he got his first DSLR camera, the Canon Rebel. Since then, he's taken tens of thousands of photos. His favorite handheld camera these days is his Google Pixel 6 XL. He loves taking photos of nature and his kids. In 2016, he bought his first drone, the Mavic Pro. Taking photos from the air is an amazing perspective, and he loves to take his drone while traveling.
Related Articles:
What Is the Best Binocular Magnification for Hunting? Optical Features Explained
How to Clean a Refractor Telescope: Step-by-Step Guide
How to Clean a Telescope Eyepiece: Step-by-Step Guide
How to Clean a Rifle Scope: 8 Expert Tips
Monocular vs Telescope: Differences Explained (With Pictures)
What Is a Monocular Used For? 8 Common Functions
How to Clean a Telescope Mirror: 8 Expert Tips
Brightfield vs Phase Contrast Microscopy: The Differences Explained
